How UPenn Researchers Predicted Heart Transplant Rejection datma.BASE
Heart disease remains the leading cause of death in the US, often necessitating critical and expensive procedures like heart transplants. However, about 12% of recipients face rejection within the first year. Accurate and early prediction of rejection could significantly improve patient outcomes and alleviate the emotional and financial stresses related to organ rejection. While current decisions are informed by histopathology images, the addition of different data types, such as molecular markers, shows promise for better predictions.
At datma, we have been privileged to support researchers in identifying new biomarkers to predict heart transplant rejection. Our multifaceted platform, datma.BASE, is designed for storing and analyzing multimodal data. Additionally, our clinical insights tool, datma.360, has been customized to support digital pathology data visualization and annotation. These technologies play a crucial role in advancing medicine through the development of Machine Learning and AI models.
Transforming Digital Pathology with datma.BASE
This research project spans several years and multiple phases. Phase 1 focuses on creating an annotated digital pathology image dataset to pioneer AI model development. To this end, datma has curated over 350 digital heart tissue images. These images have been processed and uploaded to datma.BASE’s “PathDS” platform, which organizes digital pathology images in a hierarchical structure. Each layer provides a higher resolution view than the last, and the images are divided into “tiles”, enabling selective viewing without the need to load the entire image into memory.
As a cloud-native solution, PathDS excels in storing these images efficiently, ensuring quick access and supporting essential tasks such as image annotation and labeling for both AI analysis and display.
datma.360: A Multimodal Visualization Tool
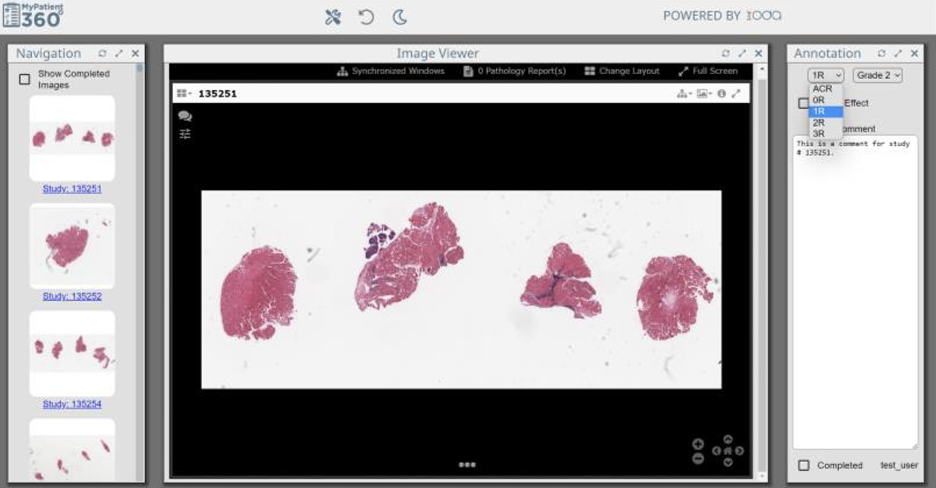
datma.360 offers pathologists intuitive image annotation and visualization capabilities. This enhances efficiency by facilitating swift navigation across multiple images, enabling streamlined reporting on key biomarkers like ACR and AbMR. Furthermore, pathologists can indicate the presence of Quilty lesions, a key indicator of heart transplant rejection. Finally, a free-text input form allows for recording crucial observations and insights for future reference.
The Road Ahead: Integrating Deep Learning Models
The long-term goal of this project is to develop advanced AI models that accurately predict heart transplant rejection. Pathologist annotations on these images provide invaluable training data for identifying morphological features associated with rejection.
While initially focusing on digital pathology, future stages will integrate advanced genomic data like transcriptomics. This multimodal approach promises to greatly enhance our AI models’ accuracy and effectiveness.
Conclusion
At datma, we are leading the way with our innovative platforms—datma.BASE and datma.360—in pioneering new methods to predict heart transplant rejection. Our current work in aggregating and annotating digital pathology images sets the stage for advanced AI model development. With our ongoing efforts and the future integration of our data insights platform we aim to improve patient outcomes and reduce the occurrences of heart transplant rejection, marking a new chapter in medical research.
The application of datma.BASE extends beyond the realm of heart transplants as it has also been used extensively in Oncology. We are also exploring its potential in other areas, demonstrating the versatility and expanding capabilities of our platform in advancing predictive healthcare solutions. With datma’s suite of solutions, we continue to create a comprehensive ecosystem that empowers physicians and researchers to make more informed decisions for improved patient outcomes.